Abstract
Background: Primary central nervous system lymphoma (PCNSL) is a rare and aggressive subtype of B-cell lymphoma with poor survival. Genetic alterations often occur in the chronic active B-cell receptor signaling which mediate the response to BTK inhibition (BTKi) in PCNSL. However, the efficacy of traditional high-dose methotrexate-based regimen as a first-line treatment was suboptimal with a short effective remission time and low response rate. Therefore, we initiated this phase II trial to investigate the efficacy of novel induction combination of high-dose methotrexate (HD-MTX) plus ibrutinib and temozolomide (MIT), in the treatment of newly diagnosed PCNSL for the first time (ClinicalTrials.gov identifier: NCT04514393).
Methods: Patients aged 18-75 years, newly diagnosed PCNSL and at least one measurable or evaluable lesion were enrolled. The MIT induction treatment included six cycles of HD-MTX (3.5 g/m 2, every three weeks), ibrutinib (560 mg/d, after HD-MTX clearance), and temozolomide (150 mg/m2 d1-d5, every three weeks). After the completion of the six cycles of MIT induction treatment, ASCT consolidation (only for patients < 65 years) or daily ibrutinib maintenance (560 mg/d) were administered up to two years, or until disease progression, intolerable toxicity, or death. Treatment response was evaluated by brain MRI and FDG-PET and/or cerebrospinal fluid (CSF) examination every two cycles of MIT treatment. The primary objective of this study was to evaluate the overall response rate (ORR) of MIT induction treatment. The safety profile of MIT was also investigated. At the same time, next generation sequencing (NGS) of baseline tissues and dynamic monitoring with CSF/ plasma ctDNA were performed by Nanjing Geneseeq Technology Inc.
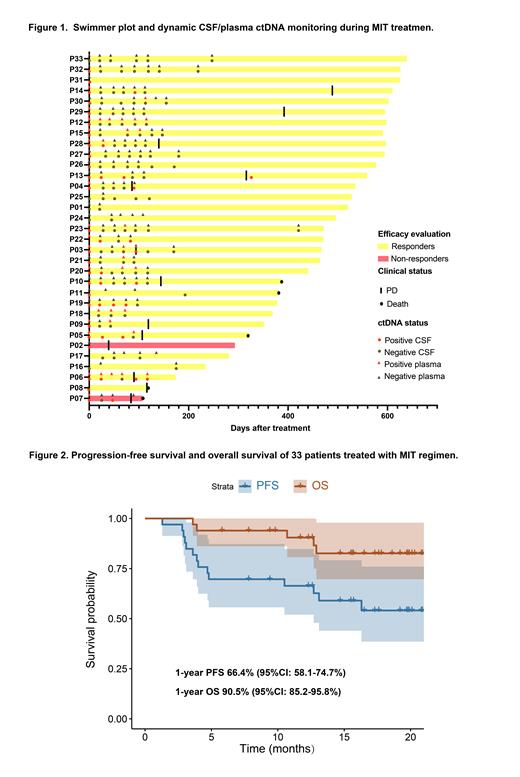
Results: Between July 2021 and October 2022, thirty-three patients were enrolled in this open-label, multi-center phase II prospective clinical study. The median age was 56 years (range: 27-75) and 17 of them were male. Eye involvement occurred in 5 patients (15%). Twenty-five patients (76%) had multiple cerebral lesions. The best ORR was 94% and complete response rate was 73%. The median follow-up was 17.5 months (range: 5.8-21.3 months) for survivors. Twenty-three patients underwent ibrutinib maintenance after 6 cycles of induction therapy. The swimmer plot of the 33 patients was shown in Figure 1. The 1-year progression-free survival was 66.4% (95%CI: 58.1-74.7%) and overall survival was 90.5% (95%CI: 85.2-95.8%) (Figure 2). Treatment-related toxicities were acceptable, with grade 3-4 neutropenia occurring in 9%, thrombocytopenia in 6%, subdural hematoma (SH) in 6%, and atrial fibrillation in 3%. The 2 patients with SH recovered following discontinuation of ibrutinib. No fungal infections and treatment-related deaths were observed. NGS of Tissue/CSF was tested in 30 patients, 19 (63%) of whom were classified as MCD subtype, 2 (7%) as BN2 subtype. The mutational profiles of baseline tissue and/or CSF samples revealed that PIM1 and MYD88 mutations were present in 75.9% and 65.5% of the entire cohort followed by BTG2 (55.2%) and CD79B (51.7%). Patients with dynamic CSF ctDNA monitoring during MIT treatment were analyzed (Figure 1). The consistence of ctDNA clearance in CSF/plasma samples and imaging complete remission was observed. Patients with clearance of ctDNA in the CSF after 2 cycles of MIT treatment achieved better PFS.
Conclusions: The novel induction treatment of MIT achieved encouraging responses in newly diagnosed PCNSL patients with acceptable toxicities. The early clearance of ctDNA in CSF may be related to favorable survival.
Disclosures
No relevant conflicts of interest to declare.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal